Mental health care for all
We build solutions to make effective mental health care accessible to everyone – anytime, anywhere.
Most people in need of mental health support don’t receive it
In addition to stigma, access to care and financial barriers keep millions from accessing the quality care they need.
65% of US adults with mental health conditions don’t receive treatment.1
Average wait time to see a licensed mental health provider.2
of adults with a mental health condition don’t receive necessary care because they can’t afford it.3
It’s time for accessible mental health care that works
Big Health empowers people and organizations everywhere to take control of their mental health with our comprehensive platform and proven digital programs.
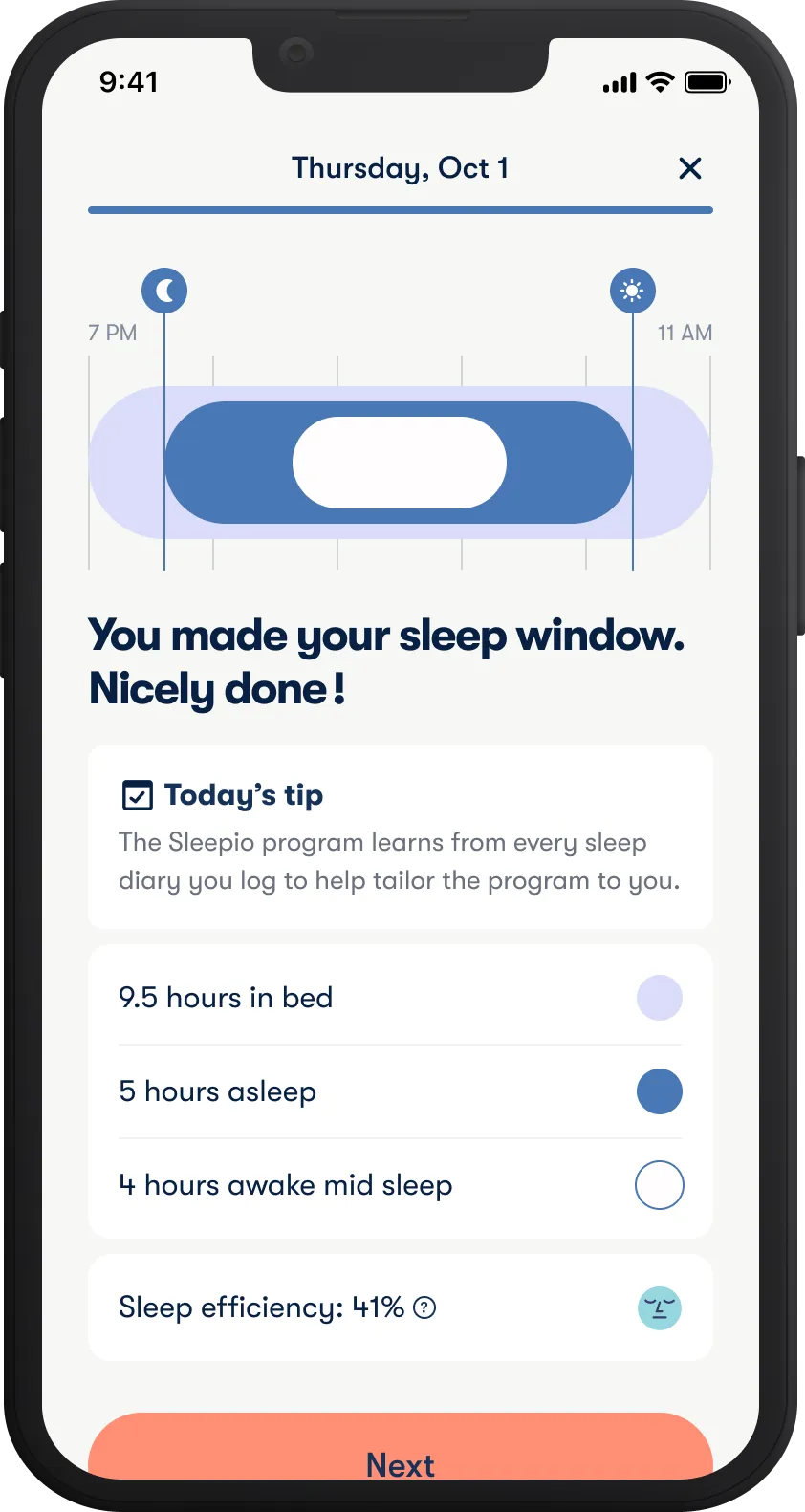
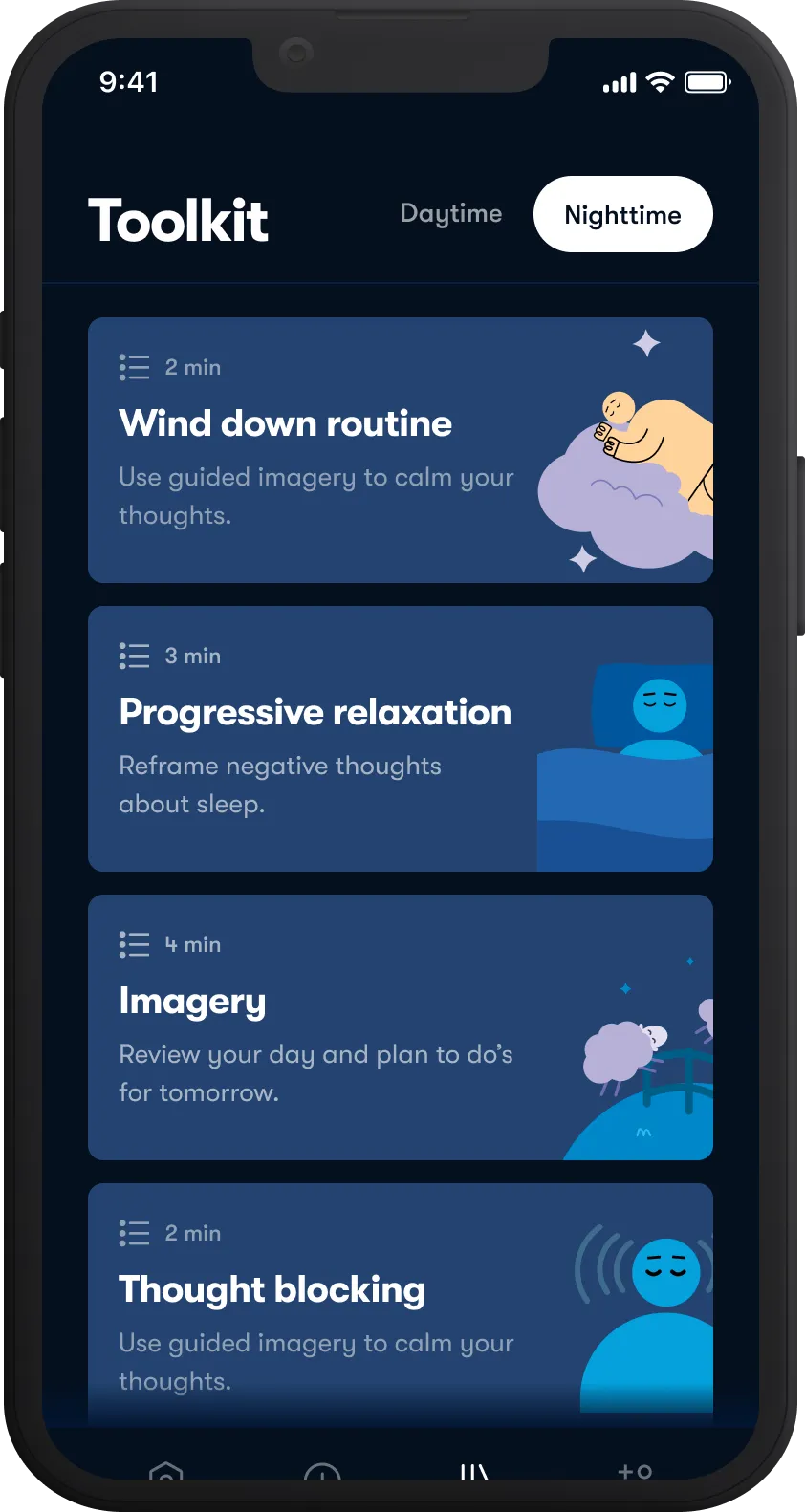
Get better sleep
Improve sleep quality with Sleepio, our digital program for insomnia.
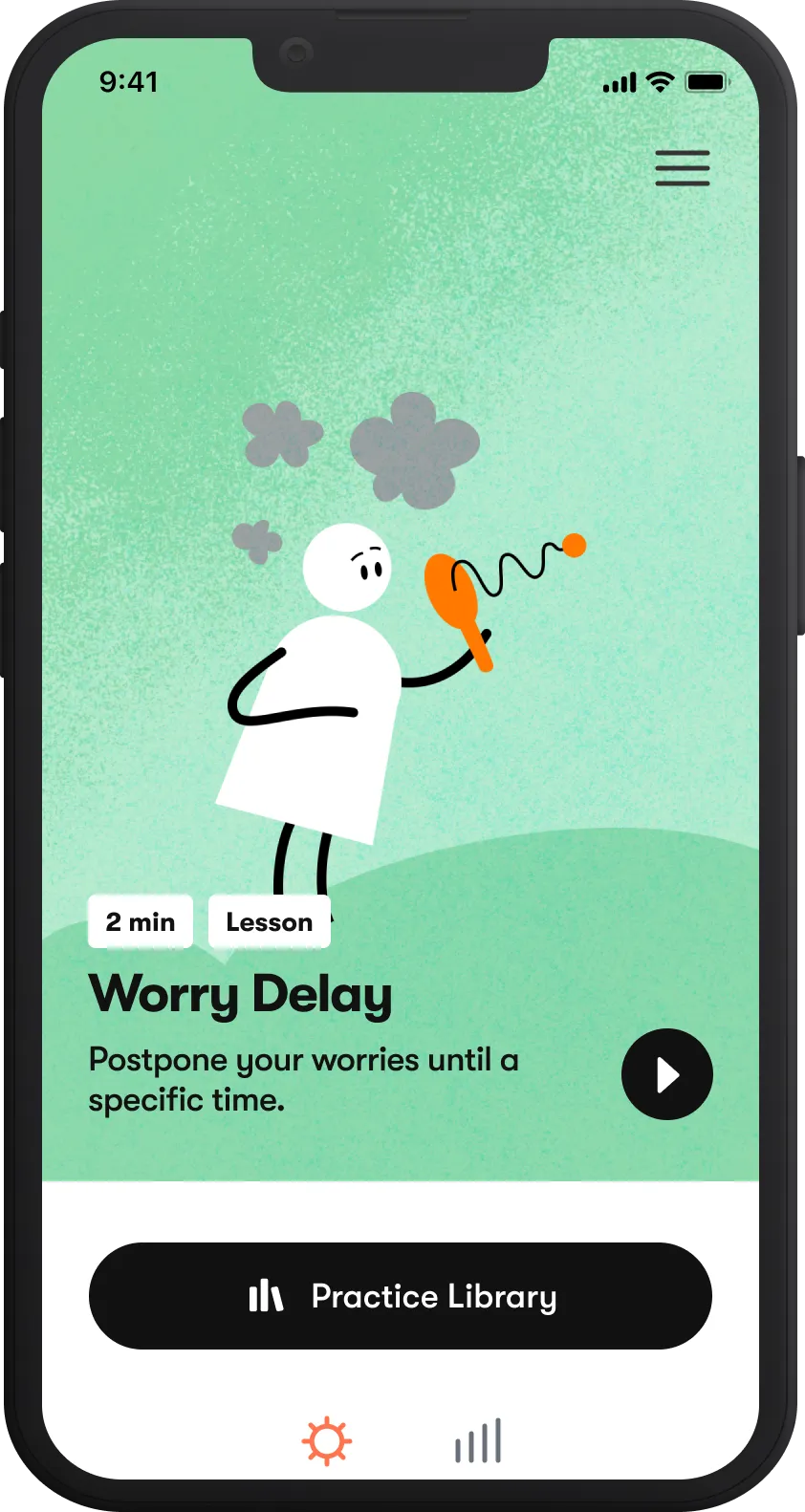
Tackle anxiety
Overcome stress and worries with Daylight, our digital program for anxiety.
Improve mood
Enhance well-being with Spark Direct, our digital program for depression.
Restore mental health
Target a range of individual needs with customized support and live care navigation.
.webp)
Who we serve
Improving productivity in automotive workers with quality sleep
A case study examining Sleepio adoption and outcomes in employees of an integrated automotive services company.
Ready to help bring millions back to good mental health?
1. Reinbert et al (2021). “The State of Mental Health in America 2022.” Mental Health America, Alexandria VA.
2. Association for Behavioral Healthcare (2022). “Outpatient mental health access and workforce crisis issue brief”
3. National Alliance on Mental Illness. (2021). “2021 Mood Disorder Survey”
During the COVID-19 public health emergency, Sleepio and Daylight are being made available as treatments for insomnia disorder and generalized anxiety disorder (GAD), respectively, without a prescription. Sleepio and Daylight have not been cleared by the U.S. Food and Drug Administration (FDA) for the treatment of insomnia disorder and GAD, respectively. Users are directed to not make any changes to their prescribed medication or other type of medical treatment without seeking professional medical advice.
Spark Direct is a digital program that may help individuals live well with major depressive disorder (MDD) and symptoms of depression by providing them with cognitive and behavioral techniques that can improve mood. Spark Direct has not been reviewed or approved by the Food & Drug Administration and is not intended to diagnose or treat any medical condition. Please read the instructions for use.
DOC-737 Effective Mar 2024
.png)